In recent years, Medical Engineering has gained in significance and has proven to be a recession-proof and innovative sector. We are all getting older and healthcare – and medical progress – require competent, well-trained specialists. With our consecutive Master's degree program Medical Devices - Research and Development (MMD), you can make a contribution to medical progress and take on responsibility for people's future and health by developing and improving medical equipment.
The degree program is structured in three semesters and follows on from a successfully-completed Bachelor’s degree or Diploma. It aims to prepare you for challenging roles in science, research and development as well as in related areas. The course comprises the module groups “Development of Medical Products”, “Engineering Science”, “Medical Engineering”, “Simulation and Modeling” and “Management”. Depending on your preferences and previous experience, you can adapt your course individually with a project and by selecting elective modules to further your knowledge in a particular direction. The laboratory and project work for the course are incorporated into industrial and research projects. This ensures that the degree program stays closely-aligned with the latest research. Quality management of teaching and research and regular evaluations guarantee your education will be of a high standard.
Apply now
Video
The following video
will show you what is special about the degree program MMD, and which characteristics you as a student will need for it.
What to expect
The
focus of our course is in line with industrial requirements,
and includes approval of medical products
(e.g. the current European Medical Device Directive, FDA,...), modern,
computer-assisted development techniques (e.g.
image processing, FEM,..), as well as a Master's project running parallel to lectures (e.g.
project management, design of experiments, statistics and management).
Our course content is closely aligned with the research topics "Biotechnology Instrumentation", "Biomechatronics"
and "Diagnostics-
and Therapy Systems, E-Health". Practically-oriented projects and theses
withresearch partners from industry will give you the best possible preparation for your career.
You can also do a doctoral degreeif
your grades are good enough.
To help make your decision easier,
the interview will be combined with an information event and a tour of our laboratories.
You will also get the chance to chat with our professors, staff and students.
We look forward to receiving your
application.
FAQs
Do you still have questions about applying? Find all the answers in the Online Application FAQs.
Prerequisites
You can apply for the Master’s degree program MMD if you have an above-average qualification from a technically-oriented degree course. The specialization in Medical Devices - Research and Development requires fundamental knowledge of this area, which must be acquired via additional courses if necessary.
Course schedule
The three-semester full-time course has a modular structure. You can select the elective modules and the alternative module from a catalog, in line with your personal interests and preferences. Additional classes in engineering, science and medicine are offered during the semester, enabling you to fill any gaps in your knowledge. After successful completion of the degree program, you will be awarded the academic degree title “Master of Engineering”.
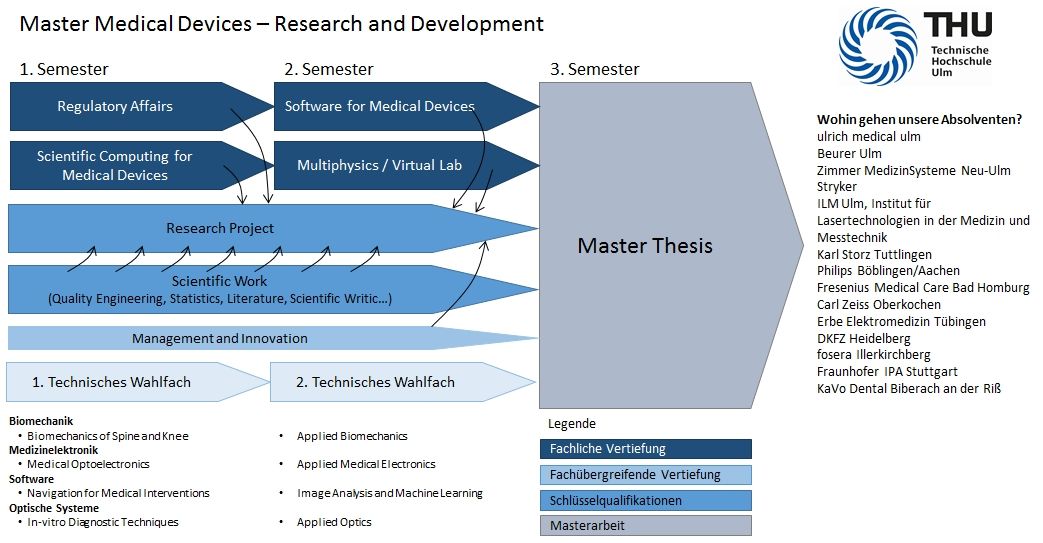
Module list
Career prospects
The market prospects for medical products are increasing: due to demographic developments in industrialized countries and due to increased industrialization in emerging economies – resulting in growing demands upon the healthcare system. The German medical engineering industry is a global leader. Highly-qualified experts and managers with specialist knowledge are always sought-after here.
The internationally-recognized Master’s qualification meets these demands. With this Master’s qualification, you will improve your career prospects and have an excellent chance of finding a job in research and development, as a project leader or manager in an international organization. Alongside development and innovation management, potential careers include system development and medical engineering management, IT processes and imaging techniques, development and research in medical engineering, simulation-based development processes, diagnostics, and process and quality management. Furthermore, the Master’s qualification enables you to do a doctoral degree and qualifies you for entry into the civil service on the “höheren Dienst” career path.
Certified quality
Certified quality
By deciding to undertake a quality-assured degree program at Ulm University of Applied Sciences you are well prepared for the future. All the Bachelor's and Master's degree courses are accredited. As part of the accreditation, the feasibility of the degree program, the contents of the course of study and the suitability of the course's graduates for the requirements of their future careers are assessed. This means that you will receive a degree qualification which is recognized and quality-certified, which will help you with the recognition of your degree program – both nationally and internationally. The accreditation of the degree programs at the Ulm University of Applied Sciences offers students and employers a reliable guide regarding the quality of the degree programs.
Downloads
Course and examination regulations
Admissions regulations
Overview of all course and examination regulations
|
The course and examination regulations define the conditions and legal requirements for a proper degree course.
The regulations for the admissions procedure for Master’s degree programs define the conditions and the procedure for admission to the Master's degree program Medical Devices - Research and Development (MMD).
Please note: if you are already a registered student at Ulm University of Applied Sciences, it might be that a different version of the course and examination regulations apply for you. The course and examination regulations which were in effect when you started your course are the ones which apply. If in doubt, please contact your Studiendekan.
|
| Module handbook |
The module handbook describes the modules belonging to the degree course, their content, the intended learning outcomes, and the form of academic assessment.
Please note: the module handbook / module list is updated by March 1 for the relevant summer semester and by September 1 for the relevant winter semester, and is valid from this point onwards. Please contact your program coordinator if you have questions regarding earlier module descriptions.
|